Realizing High Performance Four-Electron Zinc-Iodine Batteries with Acidic Eutectic Electrolyte
Citation
Yuhuan Yan, Yucong Jiao*, and Peiyi Wu*. Realizing High Performance Four-Electron Zinc-Iodine Batteries with Acidic Eutectic Electrolyte. Angew. Chem. Int. Ed. 2025, 64, e202515633.
Abstract
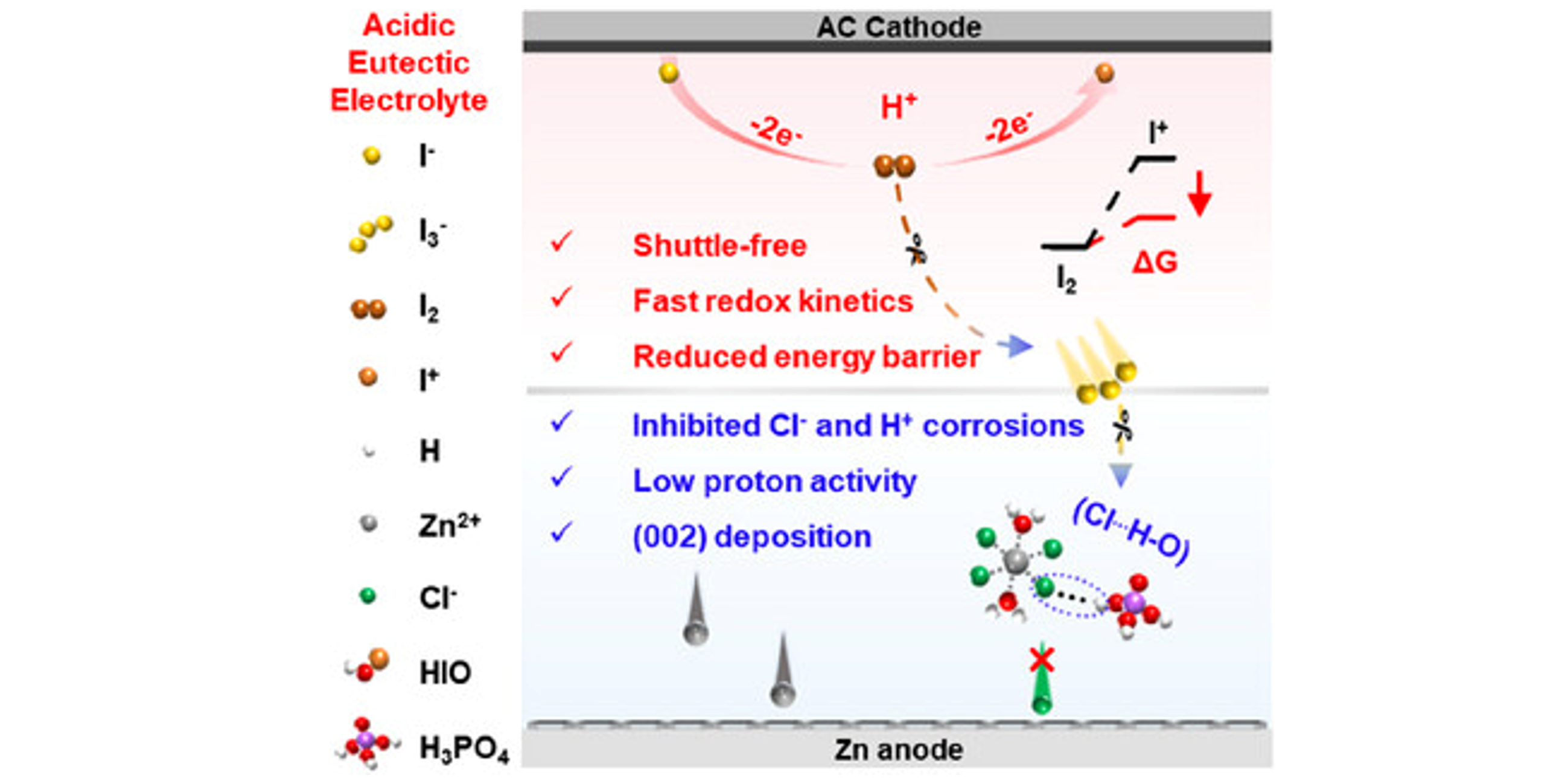
Achieving multi-electron redox reaction via halide ions and protons is promising for Zn─I2 batteries, yet their practical application is hindered by halide ion and proton corrosion, sluggish iodine redox kinetics and poor redox reversibility. Here, a deep eutectic solvent (ZPDES) composed with concentrated ZnCl2 and H3PO4 is engineered as the electrolyte for high performance Zn-I2 batteries. The chloride atom (Cl) and active hydrogen of H3PO4 in DES form hydrogen bonds (Cl⋯H─O) to mitigate the corrosion of Cl− and protons. Therefore, while achieving four-electron transfer by Cl−, the protons in DES can accelerate redox reaction kinetics and reduce I3− formation for improved reversibility. Moreover, the proton can promote the (002) texture formation and reduce by-products on Zn anode. As a result, the Zn─I2 battery with ZPDES delivers a high specific capacity of 576 mA h g−1 at 0.5 A g−1 after 320 cycles, and maintains a capacity retention of 100% over 20,000 cycles at 7 A g−1.