Exploring the Hydrogen-Bond Structures in Sodium Alginate through Two-Dimensional Correlation Infrared Spectroscopy
Citation
Lei Hou, and Peiyi Wu*. Exploring the Hydrogen-Bond Structures in Sodium Alginate through Two-Dimensional Correlation Infrared Spectroscopy. Carbohydr. Polym. 2019, 205, 420-426.
Abstract
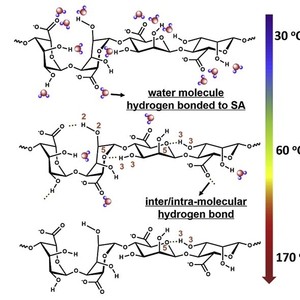
In this paper, the heat-induced hydrogen bonding evolution in the sodium alginate (SA) film is explored mainly by FTIR spectroscopy in combination with perturbation correlation moving window (PCMW) technique and 2D correlation spectroscopy (2Dcos). Due to the strong hydrophilicity, hydrogen bonds formed between water molecules and polar groups (including OH and COO−) along SA chains hold an predominant role at room temperature, which is believed to disrupt the inter/intra-molecular hydrogen bonding in SA chains. During heating from 30 to 60 °C, most water molecules evaporate abruptly, resulting in the formation of inter/intra-molecular hydrogen bonding within SA chains, such as O3H3…O5 and O2H2…O = C–O−. Upon further heating form 60 to 170 °C, both inter/intra-molecular hydrogen bonding in SA chains and hydrogen bonds of SA with water molecules are gradually broken, leading to the appearance of relatively free COH and COO− groups.