Multiple Interactions Regulated Phase Transition Behavior of Thermo-Responsive Copolymers...
Citation
Yingna Zhang, Hui Tang*, and Peiyi Wu*. Multiple Interactions Regulated Phase Transition Behavior of Thermo-Responsive Copolymers Containing Cationic Poly(Ionic Liquid)s. Phys. Chem. Chem. Phys. 2017, 19, 30804-30813.
Abstract
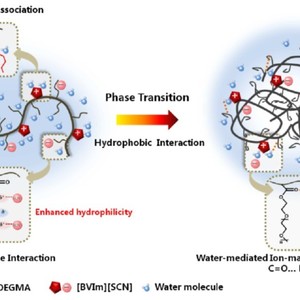
The effect of multiple interactions including anion-macromolecule interaction, water-mediated ion-macromolecule interaction and hydrophobic interaction on the phase transition behaviors of random copolymers P(OEGMA-co-BVIm[X]) comprising oligo(ethylene glycol)methacrylate (OEGMA) and imidazolium-based ionic liquids was investigated in the present study. Temperature-variable 1H NMR and FT-IR investigations demonstrated that the hydration of CH2 side chains in P(OEGMA-co-BVIm[SCN]) was enhanced due to the anion-dipole interaction between chaotropic anion SCN- and CH2 groups, and the dehydration of C=O groups served as the driving force of the phase transition. Especially, the formation of C=O…D2O-PIL hydrogen bonds were preferred in P(OEGMA-co-BVIm[SCN]) during the phase transition process considering the interaction of IL-D2O associations and C=O groups. This water-mediated ion-macromolecule interaction acted as “linkage” among polymers, resulting in the gradual dehydration of copolymers and the formation of stable small size micelles. As for P(OEGMA-co-BVIm[NTf2]), water molecules were sequentially squeezed out of the polymer chains upon heating and the self-aggregation of polymer chains was carried out through hydrophobic interaction between OEGMA side chains with IL segments wrapped in the aggregates.