Spectral Interpretation of Thermally Irreversible Recovery of Poly(N-isopropylacrylamide-co-acrylic acid) Hydrogel
Abstract
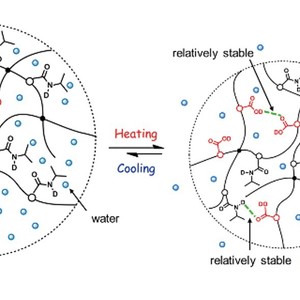
The thermally induced volume phase transition process of poly(N-isopropylacrylamide-co-acrylic acid) (PNIPAM-co-AA) hydrogel is studied using FT-IR spectroscopy in combination with the perturbation correlation moving window (PCMW) technique and two-dimensional correlation spectroscopy (2Dcos) analysis. According to PCMW spectra analysis, an elevation of volume phase transition temperature (VPTT) due to an extra equilibrium of repulsive electrostatic interactions of acrylic acid moieties in hydrogel from 34 °C to ca. physiological temperature (37 °C) is determined. 2Dcos helps us to conclude that the dehydration of hydrogel responds earlier in the process of network collapse than hydrogen bond variations of AA and NIPAM moieties during heating, while the hydrogen bonds of NIPAM and AA moieties change before the network swelling in the cooling process. Furthermore, relatively stable inner hydrogen bonds of AA moieties restrict the complete expansion of PNIPAM-co-AA hydrogel, resulting in a unique irreversible recovery during cooling.

<<全文链接>>