Integrated Microdynamics Mechanism of the Thermal-Induced Phase Separation Behavior of Poly(vinyl methyl ether)...
Abstract
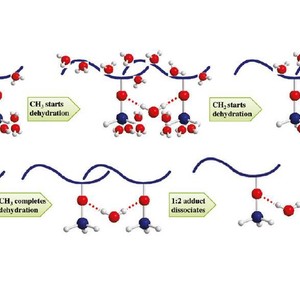
The thermal behavior of a poly(vinyl methyl ether) (PVME) aqueous solution (30 wt %) during a heating-and-cooling cycle is studied using FTIR spectroscopy in combination with 2D correlation analysis. The FTIR spectral data of O−H, CH3−O, and C−H stretching vibration regions provide detailed changes of hydrophilic and hydrophobic groups of PVME. Hydrogen bonds between hydrophilic groups and water and hydration interactions between hydrophobic groups and water are confirmed to be completely reversible in the heating-and-cooling cycle. Two-dimensional correlation method helps us to understand the microdynamics mechanism of phase separation behavior of PVME 30 wt % aqueous solution. During the heating process, the initially hydrated CH3 groups start to dehydrate as the first action of phase separation, and the initially hydrated CH2 groups follow to start their dehydration; interestingly, water molecules leave CH2 groups very fast, and the whole dehydration process of CH2 groups finishes even earlier than that of CH3. After hydrophobic groups finish their dehydrations, hydrogen bonds between hydrophilic group and water start to dissociate. 1:2 adducts formed between PVME and water dissociate first and transfer to the 1:1 adducts, whereas with further heating, 1:1 adducts eventually dissociate and release free water and free CH3−O. PCMW method is used as supplement to determine changing conditions of various chemical structures. During the phase separation, O−H hydrogen bond in 1:2 adduct is found to dissociate between 35.5 and 39 °C in a  style, whereas the 1:1 adduct (also considered as free water) increases between 35.5 and 39 °C in a
style, whereas the 1:1 adduct (also considered as free water) increases between 35.5 and 39 °C in a  style. Moreover, dehydration conditions of hydrophobic groups are also found. Both of the dehydrated states CH3and CH2 increase like
style. Moreover, dehydration conditions of hydrophobic groups are also found. Both of the dehydrated states CH3and CH2 increase like  .
.

<<全文链接>>