Dual-Electrode Synergistic Electrolyte Enabling Highly Reversible Multi-Electron Redox in Aqueous Zinc-Iodine Batteries
Citation
Xuan Chen#, Doudou Feng#, Yucong Jiao*, and Peiyi Wu*. Dual-Electrode Synergistic Electrolyte Enabling Highly Reversible Multi-Electron Redox in Aqueous Zinc-Iodine Batteries. Adv. Mater. 2025, 37, e13389.
Abstract
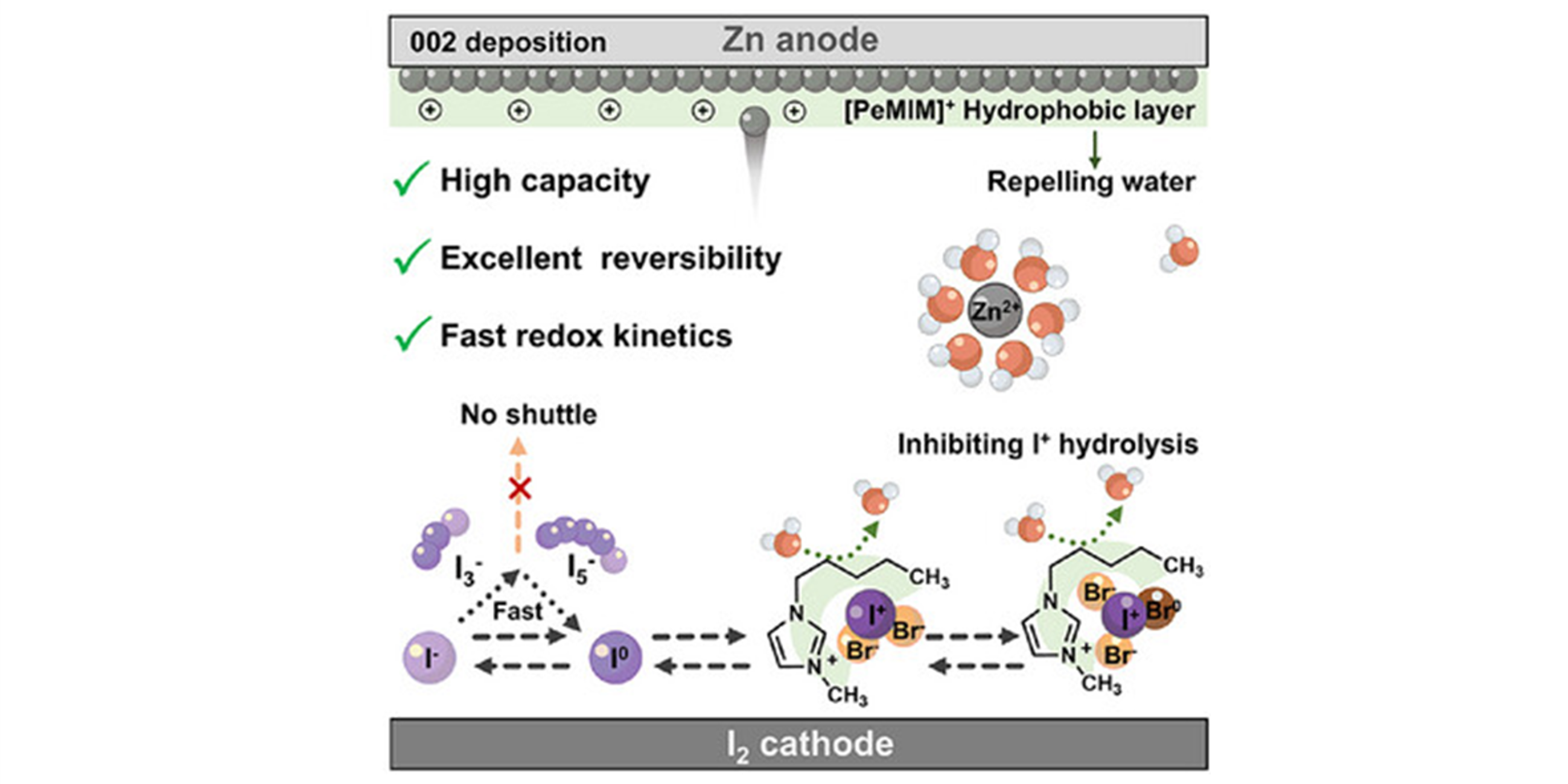
Multi-electron redox strategies offer promising approaches to achieve high energy density in aqueous Zn-iodine (Zn─I2) batteries, yet the development is impeded by unstable intermediate species, slow redox kinetics, and poor reversibility, particularly at low current densities. Herein, 1-pentyl-3-methylimidazolium bromide ([PeMIM]+Br−) is employed to develop a dual-electrode synergistic electrolyte (DESA-E), which enables the multi-electron conversion of Zn─I2 batteries with high specific capacity and long-term cycling stability. The Br− in DESA-E induces dual-halogen synergy, accelerating cascade I−/I0/I+ four-electron conversion kinetics and activating the Br−/Br0 redox reactions for ultra-high specific capacity. Meanwhile, hydrophobic [PeMIM]+ alkyl chains stabilize interhalogen intermediates, suppress I+ hydrolysis, and guide Zn deposition along the (002) plane via electrostatic effect. Consequently, the DESA-E enables Zn─I2 batteries with a specific capacity of 557 mA h g−1 after 500 cycles at 0.5 A g−1 with an average coulombic efficiency of 99.97%, and maintains a low degradation rate of 0.00055% per cycle over 60 000 cycles at 8 A g−1. This work presents a facile and cost-effective electrolyte design to enable durable multi-electron Zn─I2 batteries for high-energy-density systems.