Understanding the Thermosensitivity of POEGA-Based Star Polymers: LCST-Type Transition in Water vs. UCST-Type...
Abstract
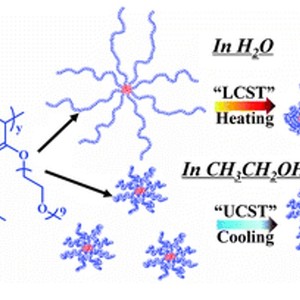
The lower critical solution temperature (LCST) transition in water and the upper critical solution temperature (UCST) transition in ethanol of poly(oligo(ethylene glycol) acrylate) (POEGA)-based core cross-linked star (CCS) polymers have been investigated and compared by employing turbidity, dynamic light scattering (DLS), 1H NMR and FTIR measurements. Macroscopic phase transitions in water and in ethanol were observed to occur when passing through the transition temperature, as revealed by DLS and turbidity measurements. Analysis by IR indicated that the interactions between the polymer chains and solvent molecules in water are stronger than those in ethanol such that the CCS polymer arm chains in water adopt more extended conformations. Moreover, hydrophobic interaction among the aliphatic groups plays a predominant role in the LCST-type transition in water whereas weak solvation of the polymer chains results in the UCST-type transition in ethanol. Additionally, the LCST-type transition in water was observed to be much more abrupt and complete than the UCST-type transition in ethanol, as suggested by 1H NMR and IR at the molecular level. Finally, an abnormal “forced hydration” phenomenon was observed during the LCST transition upon heating. This study provides a detailed understanding of the subtle distinctions between the thermal transitions of CCS polymers in two commonly used solvents, which may be useful to guide future materials design for a wide range of applications.

<<全文链接>>