Fast Proton Conduction in Denatured Bovine Serum Albumin Coated Nafion Membranes
Citation
Wei Jia, and Peiyi Wu*. Fast Proton Conduction in Denatured Bovine Serum Albumin Coated Nafion Membranes. ACS Appl. Mater. Interfaces 2018, 10, 39768-39776.
Abstract
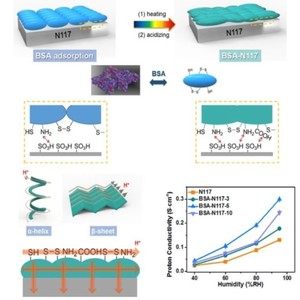
BSA (bovine serum albumin) is a globular soluble protein, which has been extensively used in biochemical engineering. BSA materials possess abundant hydrophilic charged amino acids, H-bonded networks and various secondary structures, which has great potential in facilitating proton transfer. Herein, BSA-N117 (BSA-Nafion 117) membranes are conveniently and eco-friendly prepared by utilizing the adsorption and denaturation of BSA on the Nafion117 surface. The morphology and secondary structures of the BSA layer are studied with FE-SEM, AFM, and FTIR. BSA-N117 membranes show highly increased proton conductivity under various conditions, which could be attributed to the improved wettability, water uptake and the denaturation of BSA. The in-plane proton conductivity of BSA-N117-5 reaches 0.3 and 0.06 S cm-1 under 80 oC-95 %RH and 100 oC – 40 %RH, respectively. The denaturation of BSA leads to the unfolding of α-Helix structures and the formation of β-sheet structures. β-Sheet structures are more beneficial to proton conduction since β-Sheet structures have stronger interactions with water molecules and protons could transport more directly in the parallel H-bonded network. Moreover, the denatured BSA modification layer could effectively help BSA-N117 membranes to possess higher selectivity and overcome the “trade-off” effect between proton conductivity and methanol resistance. The methanol permeability of BSA-N117 membranes is one order of magnitude lower than that of Nafion117.