Block Length-Dependent Phase Transition of Poly(N-Isopropylacrylamide)-b-Poly(2-Isopropyl-2-Oxazoline) Diblock Copolymer
Citation
Yuanyuan Zhou, and Peiyi Wu*. Block Length-Dependent Phase Transition of Poly(N-Isopropylacrylamide)-B-Poly(2-Isopropyl-2-Oxazoline) Diblock Copolymer in Water. Polymer 2018, 153, 250-261.
Abstract
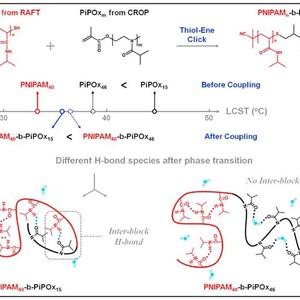
Poly(N-isopropylacrylamide)-b-Poly(2-isopropyl-2-oxazoline) diblock copolymers have been synthesized via a facile thiol-ene click approach between reversible addition-fragmentation chain transfer (RAFT) polymerized PNIPAM and cationic ring-opening polymerized PiPOx homopolymers. Based on temperature-variable Fourier transform infrared (FTIR) spectra and two-dimensional correlation analysis, the diblock copolymers are proved to exhibit block length-dependent phase transition mechanisms upon heating. The existence of C=O(PiPOx)… D-N(PNIPAM) (hydrogen in H-bond is denoted by the symbol “D” rather than “H” because D2O is used as solvent instead of H2O in the present study) H-bonding interaction is highlighted in PNIPAM40-b-PiPOx15 and proven to be the driving force of the whole transition. These inter-block H-bonds act as physical cross-linking points between PNIPAM and PiPOx segments, triggering the collaborative dehydration of two components. In contrast, C=O(PiPOx) … D-N(PNIPAM) H-bond is absent in PNIPAM40-b-PiPOx46, indicating that the isolation between two blocks is enhanced upon increasing PiPOx length which makes it harder to develop inter-block H-bond. Instead, C=O(PNIPAM) … D-N(PNIPAM) H-bonds develop firstly to initiate the phase transition process, followed by the formation of C=O(PiPOx) … D-O-D … O=C(PiPOx) H-bonds, resulting in relatively independent phase transition mechanisms of these two components.