Hydrogen Bonding Reinforcement as a Strategy to Improve Upper Critical Solution Temperature of P(NAGA-co-MAA)
Citation
Wenhui Sun, Zesheng An*, and Peiyi Wu*. Hydrogen Bonding Reinforcement as a Strategy to Improve Upper Critical Solution Temperature of Poly(N-Acryloylglycinamide-Co-Methacrylic Acid). Polym. Chem. 2018, 9, 3667-3673.
Abstract
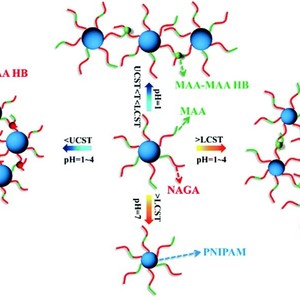
Hydrogen bonding reinforcement via copolymerization of N-acryloylglycinamide (NAGA) with methacrylic acid (MAA) is employed as a strategy to improve the upper critical solution temperature (UCST) to a biologically relevant range. P(NAGA-co-MAA) copolymers with MAA molar fraction in the range of 1–81 mol% are synthesized via reversible addition–fragmentation chain transfer (RAFT) polymerization. The UCST (3.5–37.5 °C) of the copolymers scales with MAA molar fraction in the range of 10–60 mol% when measured at pH 4 and 1 wt% concentration. Using a copolymer with a suitably high UCST as a macromolecular chain transfer agent, doubly thermoresponsive nanogels consisting of P(NAGA-co-MAA) copolymer as the shell and cross-linked poly(N-isopropylacrylamide) (PNIPAM) as the core are synthesized via RAFT aqueous dispersion polymerization. The nanogels show distinct thermal transitions with both upper and lower critical solution temperatures (UCST and LCST).