Intra-Molecular Interactions Dominating the Dehydration of a Poly(2-Isopropyl-2-oxazoline)-Based..
Abstract
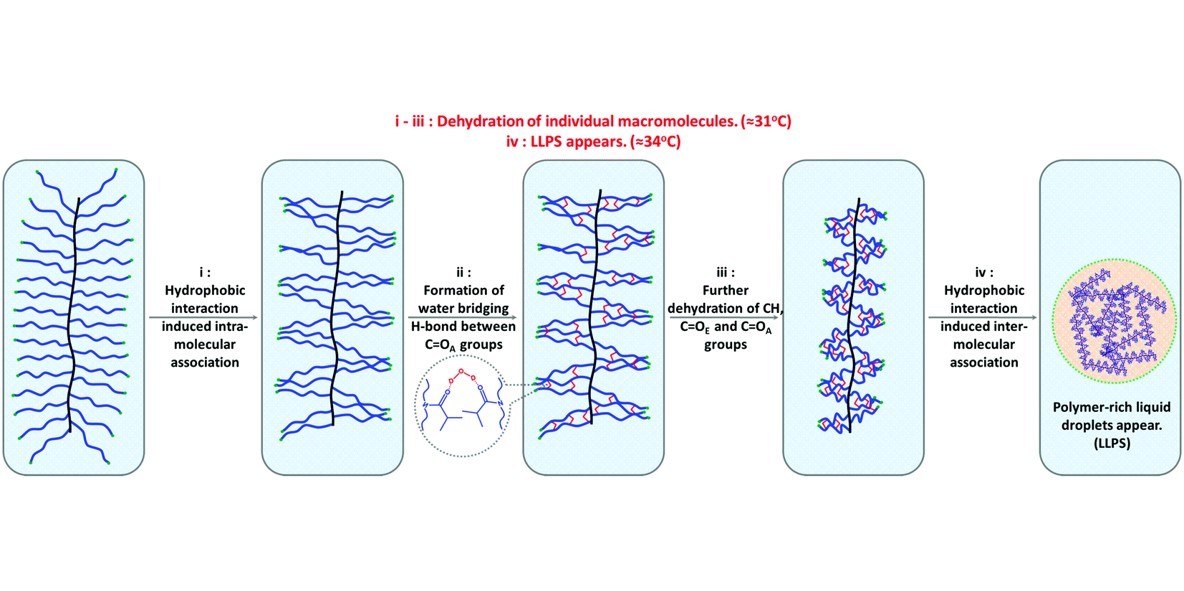
Temperature-induced phase transition together with the liquid–liquid phase separation (LLPS) phenomenon of poly(oligo(2-isopropyl-2-oxazoline)methacrylate) with the comb-shaped architecture (comb-PiPOx) in aqueous solution has been discussed at the molecular level. Differing from linear poly(2-isopropyl-2-oxazoline) (linear-PiPOx), polymer-rich liquid droplets appear at higher temperature compared with the phase transition determined by differential scanning calorimetry (DSC) in comb-PiPOx solution. As investigated using variable-temperature Fourier transform infrared (FTIR) spectra analysis, the densely grafted architecture gives rise to an intra-molecular interaction (hydrophobic interaction of alkyl groups and H-bond of carbonyl groups) dominating the dehydration process of comb-PiPOx. With temperature increment, most of the water within hydrated polymers is expelled to the outer water phase through intra-molecular association, corresponding to the transition temperature. Afterwards, the dehydration of methyl groups on side chain ends reflects the massive aggregation of polymer chains through inter-molecular association, accompanied by hysteretic LLPS.
<<全文链接>>

