Submicronic Calcite Particles with Controlled Morphology Tailored by Polymer Skeletons Via Carbonation Route...
Citation
Wei Li, Qisi Yu, and Peiyi Wu*. Submicronic Calcite Particles with Controlled Morphology Tailored by Polymer Skeletons Via Carbonation Route with Compressed or Supercritical CO2. Green Chem. 2009, 11, 1541-1549.
Abstract
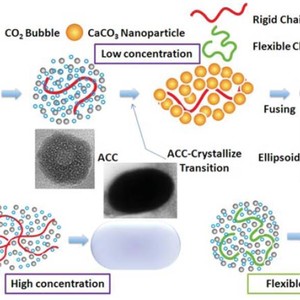
The preparation of biominerals based on CO2 fixation and regulatory effect of organic macromolecules has attracted more and more interests in recent years, inspired by the increase in technological interests in bio-inspired materials science and fabrication of useful products via an environmentally friendly process. In this paper, submicronic CaCO3particles with ellipsoidal morphology were synthesized using compressed or supercritical CO2 with sodium salt of carboxymethyl cellulose (NaCMC) as a template. By regulating some experimental parameters, including the concentration and molecular weight of NaCMC, as well as the CO2 pressure and temperature, the morphology and size of the CaCO3 particles could be effectively controlled. Besides, in contrast to the effective results of another additive, Polyacrylic Acid (PAA), our research suggested that the morphology of the synthesized particles was strongly related to the flexibility of the polymer chains, namely, the relatively rigid chains induce the formation of ellipsoidal particles while the more flexible chains would result in the spherical ones. In addition, amorphous calcium carbonate was traced as an intermediate phase during the crystallization process. Based on the experimental results, we discussed the formation mechanism of such particles: the polymer chains serve as the skeletons, then the ions attach along the chains to grow. The final morphology of CaCO3 aggregates is tailored by the flexibility of the polymer chain, while the size of the particles is related with the chain length of the polymer. In comparison with the traditional mineralization methods, here we provide a highly efficient and versatile approach to integrate the fixation of CO2 and the regulating effect of different polymer chains, to produce submicroscopic CaCO3 particles and further control their morphology and size distribution. Such an approach is facile and of potential importance as an environmentally benign industrial route to synthesize biomaterials, and it may also serve to shed light on the study of the mechanism of biomimetic mineralization.