Thermoresponsive Behavior of an LCST-Type Polymer Based on a Pyrrolidone Structure in Aqueous Solution
Abstract
Poly(3-ethyl-N-vinyl-2-pyrrolidone) (C2PVP) is a new type of thermoresponsive polymer which exhibits phase separation in water above the lower critical solution temperature (LCST) at about 26 °C. The thermoresponsive mechanism of C2PVP during the heating–cooling cycle has been investigated by means of temperature-dependent FTIR in combination with a two-dimensional correlation (2Dcos) technique and density functional theory (DFT) calculations. Compared with a secondary-amide thermoresponsive polymer such as poly(N-isopropyl acrylamide) (PNIPAM), the unusual phenomenon of an asymmetrical-to-symmetrical transition of the C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O peak shape is observed in the conventional IR spectra of tertiary-amide polymer C2PVP. 2Dcos results confirmed that two species of C
O peak shape is observed in the conventional IR spectra of tertiary-amide polymer C2PVP. 2Dcos results confirmed that two species of C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O groups (C
O groups (C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O⋯2D2O and C
O⋯2D2O and C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O⋯D2O) mainly coexist at the initial heating, and they change to the other two C
O⋯D2O) mainly coexist at the initial heating, and they change to the other two C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O groups involving the free C
O groups involving the free C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O⋯DOD⋯O
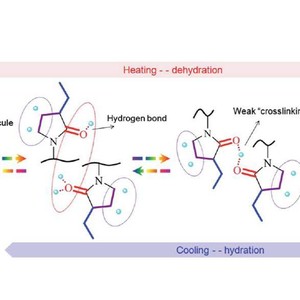
O⋯DOD⋯O![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C after heating, with the latter predominant above the LCST. A slight hysteresis is observed in the cooling process attributed to the existence of a weak “crosslinking” point originating from the structure of C
C after heating, with the latter predominant above the LCST. A slight hysteresis is observed in the cooling process attributed to the existence of a weak “crosslinking” point originating from the structure of C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O⋯DOD⋯O
O⋯DOD⋯O![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C. Based on 2Dcos results, the dehydration process of different groups could be described in the following order: ethyl groups > C
C. Based on 2Dcos results, the dehydration process of different groups could be described in the following order: ethyl groups > C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O groups > CH2 groups in ring (“>” means prior to), and the reversible sequence is observed during hydration process. In PNIPAM system, CH2 groups usually change prior to C
O groups > CH2 groups in ring (“>” means prior to), and the reversible sequence is observed during hydration process. In PNIPAM system, CH2 groups usually change prior to C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O groups, while for C2PVP the dehydrated rate of CH2 groups (in ring) lags behind that of C
O groups, while for C2PVP the dehydrated rate of CH2 groups (in ring) lags behind that of C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O groups because of the steric hindrance of pyrrolidone structures. It is worth noting that cononsolvency of C2PVP can be found in water/methanol mixed solvents. The LCST of C2PVP in water/methanol mixture shifts from 29.5 to 16 °C as the methanol volume fraction reaches 50% and the phase separation totally disappears when the content of methanol is higher than 60%.
O groups because of the steric hindrance of pyrrolidone structures. It is worth noting that cononsolvency of C2PVP can be found in water/methanol mixed solvents. The LCST of C2PVP in water/methanol mixture shifts from 29.5 to 16 °C as the methanol volume fraction reaches 50% and the phase separation totally disappears when the content of methanol is higher than 60%.

<<全文链接>>